Ketamine, the anesthetic
Ketamine is a dissociative anesthetic widely used in both human and veterinary medicine. It was derived from phencyclidine in 1962 in pursuit of a safer anesthetic with reduced hallucinogenic effects1,2. Known for its rapid action, ketamine induces a trance-like state, including pain relief, sedation, and memory loss. It has been a staple in surgical procedures and emergency medicine for several decades3. The anesthetic and analgesic properties of ketamine are primarily mediated through its antagonism of the N-methyl-D-aspartate (NMDA) receptor.
Ketamine, the antidepressant
Ketamine’s potential for treating neurological diseases such as depression, PTSD, and chronic pain has sparked significant interest within the medical and scientific communities. Administered at subanesthetic doses it offers a promising alternative to treat major depressive disorders4–6. Researchers are currently investigating the mechanisms underlying ketamine’s antidepressant effects in the brain and suggested a number of different mechanisms for its antidepressant pharmacological profile3,7,8.
Structure and metabolism
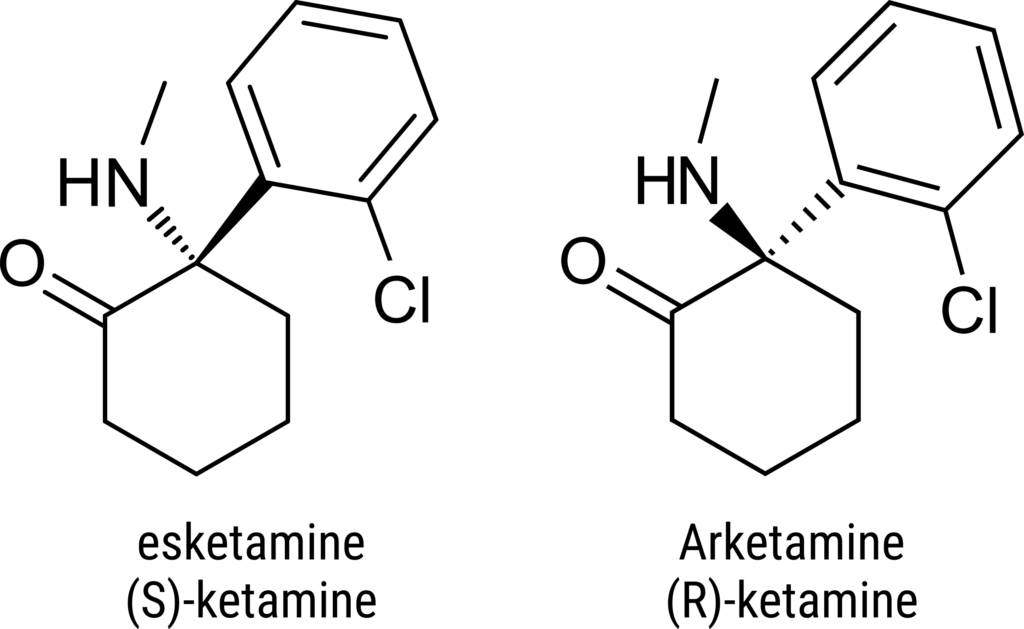
Ketamine has a chlorophenyl scaffold substituted with 2-methylamino cyclohexanone and is a chiral compound with two enantiomers: esketamine (S-ketamine) and arketamine (R-ketamine). Esketamine has higher analgesic and anesthetic effects and causes fewer psychotic and other adverse effects9.
Although both enantiomers have been shown to exhibit antidepressant outcomes10, arketamine is recognized for its longer-lasting antidepressant effects11.
Although ketamine has been used clinically for more than six decades, its metabolic pathways and pharmacological profile are still not fully understood. These gaps in knowledge make it difficult to accurately predict its effects and hinder the optimisation of its therapeutic potential. Major metabolites of ketamine have been identified12,13, and there is increasing evidence that the antidepressant effects of ketamine may be mediated by its metabolites14–17. Understanding the role and mechanisms of these metabolites may be critical to improving the efficacy of ketamine in the treatment of depression and potentially other disorders, opening new avenues for its clinical use and therapeutic refinement.
Mapping ketamine in the pig brain
A research team around Daniel Globisch at Uppsala University and Iben Lundgaard at Lund University has investigated ketamine metabolism and the distribution of ketamine metabolites in the pig brain18. The study focuses on 12 anatomically distinct brain regions, along with plasma samples from blood vessels circulating blood from and to the brain, as well as cerebrospinal fluid. Using ultraperformance liquid chromatography–mass spectrometry they map the neuro-distribution profile of ketamine, providing crucial insights into how this drug permeates and acts within different areas of the brain.
Ketamine metabolites in the pig brain
Using SIRIUS, the researchers were able to detect and identify numerous phase I and phase II metabolites of ketamine, including five metabolites that they described in vivo for the first time in this study:
- phenol-hydroxy-nKET and dihydroxy-nKET were previously detected in another study but not structurally elucidated13. This study successfully determined their structure.
- 5,6-dehydro-nKET-r shares the same molecular formula as nKET, but exhibits a distinct retention time. Further analysis confirmed its core structure as being derived from ketamine with a reduced carbonyl.
- OH-5,6-dehydro-nKET was identified as a hydroxylated analogue of 5,6-dehydro-nKET.
- OH-5,6-dehydro-nKET-Gluc is a newly identified phase II metabolite, which originated from OH-5,6-dehydro-nKET.
Neuro-distribution profile
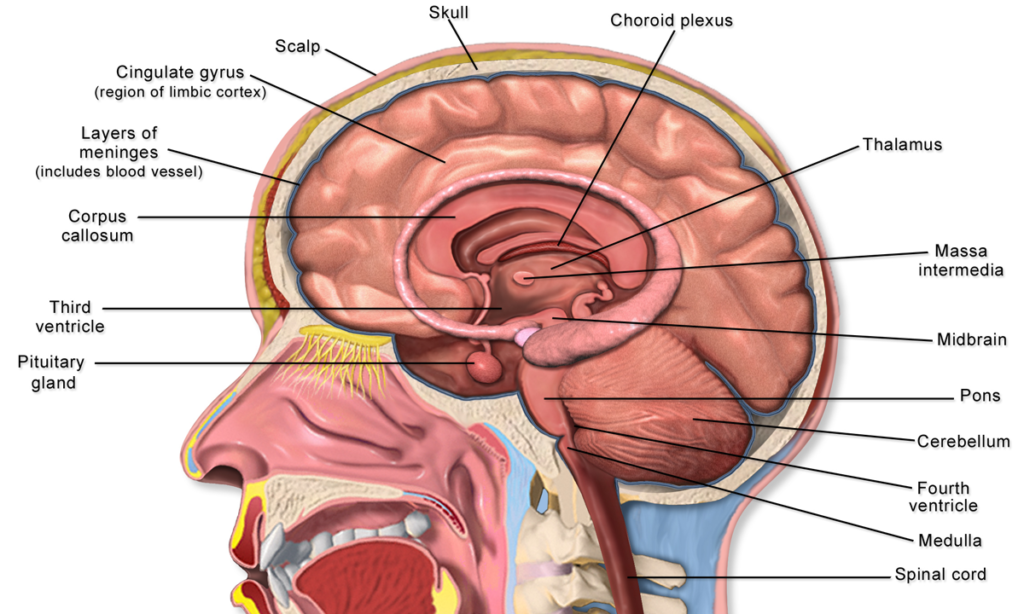
The investigation into the distribution of ketamine and its metabolites in the pig brain revealed significant regional differences, particularly in the levels of phase II metabolites. For the phase I metabolites, there was a high correlation between plasma, cerebrospinal fluid, and brain tissue, indicating their ability to traverse the blood-brain barrier effectively.
Ketamine and norketamine were found to distribute unspecifically, while their glucuronidated phase II metabolites were predominantly concentrated in the choroid plexus and the pituitary gland. Those two brain regions were the ones that differed from the other ten regions. Both regions are linked to the clearance of metabolites from the brain. The choroid plexus plays a crucial role in the production and secretion of cerebrospinal fluid. The pituitary gland, also known as the hypophysis, is an endocrine gland that controls several hormone glands in the body and plays a crucial role in regulating vital body functions and general wellbeing. The accumulation of hydrophilic glucuronidated ketamine metabolites in these regions suggests a targeted pathway that could be leveraged for developing more effective treatments for neurological disorders.
References
- 1.Peltoniemi MA, Hagelberg NM, Olkkola KT, Saari TI. Ketamine: A Review of Clinical Pharmacokinetics and Pharmacodynamics in Anesthesia and Pain Therapy. Clin Pharmacokinet. Published online March 30, 2016:1059-1077. doi:10.1007/s40262-016-0383-6
- 2.Cohen SP, Bhatia A, Buvanendran A, et al. Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Chronic Pain From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Regional Anesthesia and Pain Medicine. Published online June 2018:1. doi:10.1097/aap.0000000000000808
- 3.Wei Y, Chang L, Hashimoto K. Molecular mechanisms underlying the antidepressant actions of arketamine: beyond the NMDA receptor. Mol Psychiatry. Published online May 7, 2021:559-573. doi:10.1038/s41380-021-01121-1
- 4.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biological Psychiatry. Published online February 2000:351-354. doi:10.1016/s0006-3223(99)00230-9
- 5.Kim J, Farchione T, Potter A, Chen Q, Temple R. Esketamine for Treatment-Resistant Depression — First FDA-Approved Antidepressant in a New Class. N Engl J Med. Published online July 4, 2019:1-4. doi:10.1056/nejmp1903305
- 6.Zarate CA Jr, Brutsche NE, Ibrahim L, et al. Replication of Ketamine’s Antidepressant Efficacy in Bipolar Depression: A Randomized Controlled Add-On Trial. Biological Psychiatry. Published online June 2012:939-946. doi:10.1016/j.biopsych.2011.12.010
- 7.Yang C, Yang J, Luo A, Hashimoto K. Molecular and cellular mechanisms underlying the antidepressant effects of ketamine enantiomers and its metabolites. Transl Psychiatry. Published online November 7, 2019. doi:10.1038/s41398-019-0624-1
- 8.Lazarevic V, Yang Y, Flais I, Svenningsson P. Ketamine decreases neuronally released glutamate via retrograde stimulation of presynaptic adenosine A1 receptors. Mol Psychiatry. Published online August 11, 2021:7425-7435. doi:10.1038/s41380-021-01246-3
- 9.Andrade C. Ketamine for Depression, 3: Does Chirality Matter? J Clin Psychiatry. Published online June 28, 2017:e674-e677. doi:10.4088/jcp.17f11681
- 10.Masaki Y, Kashiwagi Y, Watabe H, Abe K. (R)‐ and (S)‐ketamine induce differential fMRI responses in conscious rats. Synapse. Published online September 5, 2019. doi:10.1002/syn.22126
- 11.Zhang K, Hashimoto K. An update on ketamine and its two enantiomers as rapid-acting antidepressants. Expert Review of Neurotherapeutics. Published online December 4, 2018:83-92. doi:10.1080/14737175.2019.1554434
- 12.Bijlsma L, Sancho JV, Hernández F, Niessen WMA. Fragmentation pathways of drugs of abuse and their metabolites based on QTOF MS/MS and MSE accurate-mass spectra. J Mass Spectrom. Published online September 2011:865-875. doi:10.1002/jms.1963
- 13.Turfus SC, Parkin MC, Cowan DA, et al. Use of Human Microsomes and Deuterated Substrates: An Alternative Approach for the Identification of Novel Metabolites of Ketamine by Mass Spectrometry. Drug Metab Dispos. Published online May 15, 2009:1769-1778. doi:10.1124/dmd.108.026328
- 14.Highland JN, Zanos P, Riggs LM, et al. Hydroxynorketamines: Pharmacology and Potential Therapeutic Applications. Dantzer R, ed. Pharmacol Rev. Published online March 5, 2021:763-791. doi:10.1124/pharmrev.120.000149
- 15.Highland JN, Morris PJ, Konrath KM, et al. Hydroxynorketamine Pharmacokinetics and Antidepressant Behavioral Effects of (2,6)- and (5R)-Methyl-(2R,6R)-hydroxynorketamines. ACS Chem Neurosci. Published online February 3, 2022:510-523. doi:10.1021/acschemneuro.1c00761
- 16.Lumsden EW, Troppoli TA, Myers SJ, et al. Antidepressant-relevant concentrations of the ketamine metabolite (2 R ,6 R )-hydroxynorketamine do not block NMDA receptor function. Proc Natl Acad Sci USA. Published online February 22, 2019:5160-5169. doi:10.1073/pnas.1816071116
- 17.Sandbaumhüter FA, Aerts JT, Theurillat R, Andrén PE, Thormann W, Jansson ET. Enantioselective CE–MS analysis of ketamine metabolites in urine. Electrophoresis. Published online December 9, 2022:125-134. doi:10.1002/elps.202200175
- 18.Vallianatou T, de Souza Anselmo C, Tsiara I, Bèchet NB, Lundgaard I, Globisch D. Identification of New Ketamine Metabolites and Their Detailed Distribution in the Mammalian Brain. ACS Chem Neurosci. Published online March 20, 2024:1335-1341. doi:10.1021/acschemneuro.4c00051








