Widespread ecological consequences of using neonicotinoids
Neonicotinoids are neuro-active insecticides chemically similar to nicotine, and have been the most widely used insecticides for nearly two decades1. By stimulating nicotinic acetylcholine receptors, they disrupt the nervous system of insects, resulting in the death or harm of a wide range of both intended and unintended insect species2. They are water-solulable and thus absorbed by plants, making them pervasive throughout the entire plant, including leaves, flowers, nectar, and pollen2. The widespread use of neonicotinoids is posing a chronic threat to pollinating insects and has been linked to a range of adverse ecological consequences3. Recognizing these risks, several countries imposed restrictions on neonicotinoid use.
Thiacloprid: a threat to health and environment
Thiacloprid belongs to the first generation of neonicotinoid insecticides. It is particularly effective in controlling a diverse range of sucking and chewing insects, such as aphids, moth scale insects, codling moths, and weevils. Notably, thiacloprid has consistently appeared as one of the most frequently detected insecticides in environmental samples4, raising concerns due to its persistent and extensive contamination, which has prompted deep apprehension about exposure to humans5. Chronic exposure to thiacloprid is associated with the potential for serious neonicotinoid toxicity, including the development of conditions like Parkinson’s and Alzheimer’s disease6, damage to human fertility7, and the risk of causing liver cancer8. In response to these concerns, the European Commission took action by banning THI from the European market.
Identifying pesticide impurities using ZODIAC
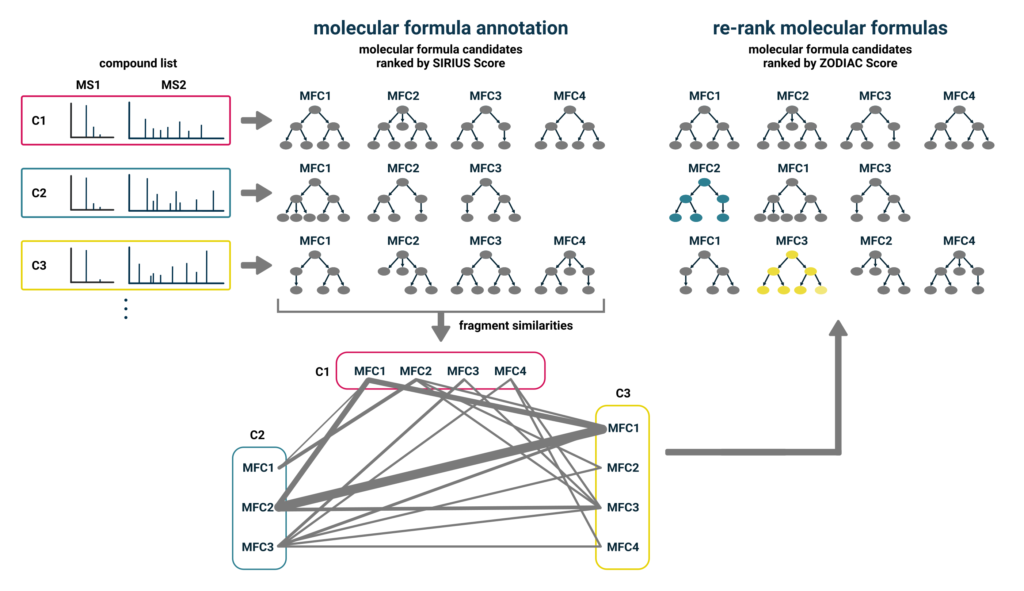
Impurities in insecticides represent a critical aspect of safety and environmental considerations. These impurities can potentially pose more severe risks than the main active compound. Variations in manufacturing processes and storage conditions can lead to significant differences in the impurity profiles. Identifying and elucidating structurally related impurities in these complex chemical mixtures is challenging9, usually combining several analytical methods. Researchers at the National Institute of Metrology, China, suggest an impurity profiling protocol10 for thiacloprid using our software SIRIUS, including ZODIAC and CSI:FingerID. ZODIAC11 is our enhanced molecular formula identification method, which is part of our SIRIUS software. SIRIUS ranks molecular formulas for each compound individually using fragmentation trees and isotope pattern analysis. ZODIAC improves these identifications by taking similarities of compounds in the dataset into account.
Thiacloprid impurities are mainly transformations of the two main moieties
Thiacloprid impurities mainly originated from precursors, by-products, or degradation products, which often exhibited a similar chemical structure. The chemical structure of thiacloprid is a composition of two moieties: 6-chloro-3-pyridinyl (CP) and 2-cyaniminothiazolidine (CIT). Fragments resulting from cleavage and decomposition of the CIT moiety were found to be widely present in the impurities helping significantly with their structural elucidation. The researchers elucidated 18 impurities and grouped them into five groups according to their structural difference: (1) precursor substitution, (2) CP moiety substitution, (3) CIT moiety substitution, (4) both CP and CIT substitution and (5) substitution within the CH2 bridge between the two moieties.
Beyond searching among known structures
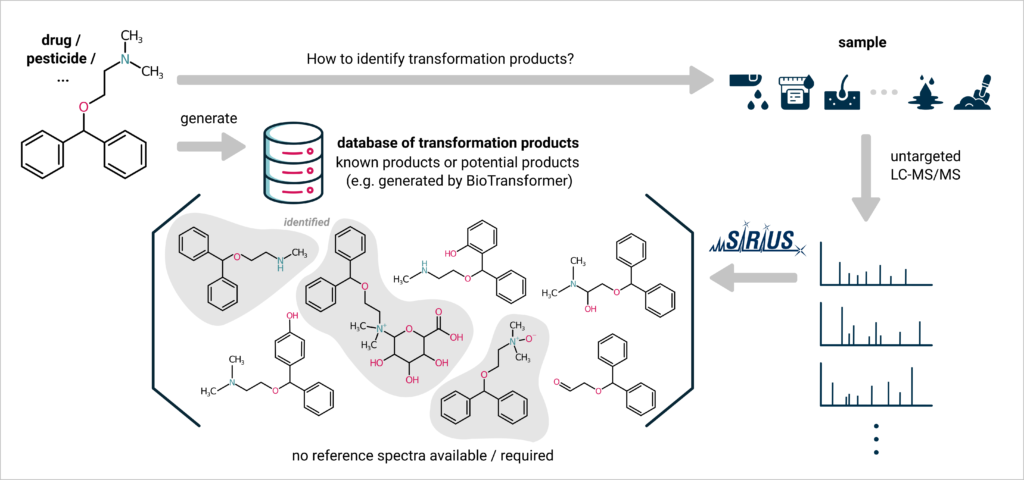
Out of the identified impurities, 14 were reported for the first time and lacked a CAS (Chemical Abstracts Service) number. Due to that, the authors claim that “CSI:FingerID […] didn’t provide much useful information for structure elucidation.”10 While this observation holds when conducting searches within public chemical databases, it’s important to note that CSI:FingerID is not restricted to any specific database. It can also be effectively employed to explore a database comprising potential structures, which are, for instance, generated by tools like BioTransformer. This approach proves especially valuable for identifiying unknown transformation products of pesticides or drugs, offering a more suitable search space for structural elucidation.
References
- 1.Casida JE. Neonicotinoids and Other Insect Nicotinic Receptor Competitive Modulators: Progress and Prospects. Annu Rev Entomol. Published online January 7, 2018:125-144. doi:10.1146/annurev-ento-020117-043042
- 2.Hladik ML, Main AR, Goulson D. Environmental Risks and Challenges Associated with Neonicotinoid Insecticides. Environ Sci Technol. Published online February 26, 2018:3329-3335. doi:10.1021/acs.est.7b06388
- 3.Wood TJ, Goulson D. The environmental risks of neonicotinoid pesticides: a review of the evidence post 2013. Environ Sci Pollut Res. Published online June 7, 2017:17285-17325. doi:10.1007/s11356-017-9240-x
- 4.Wang Z, Chen J, Zhan T, He X, Wang B. Simultaneous determination of eight neonicotinoid insecticides, fipronil and its three transformation products in sediments by continuous solvent extraction coupled with liquid chromatography-tandem mass spectrometry. Ecotoxicology and Environmental Safety. Published online February 2020:110002. doi:10.1016/j.ecoenv.2019.110002
- 5.Mahai G, Wan Y, Xia W, et al. A nationwide study of occurrence and exposure assessment of neonicotinoid insecticides and their metabolites in drinking water of China. Water Research. Published online February 2021:116630. doi:10.1016/j.watres.2020.116630
- 6.Cimino AM, Boyles AL, Thayer KA, Perry MJ. Effects of Neonicotinoid Pesticide Exposure on Human Health: A Systematic Review. Environ Health Perspect. Published online February 2017:155-162. doi:10.1289/ehp515
- 7.European Food Safety Authority (EFSA), Abdourahime H, Anastassiadou M, et al. Peer review of the pesticide risk assessment of the active substance thiacloprid. EFS2. Published online March 2019. doi:10.2903/j.efsa.2019.5595
- 8.Zhang H, Zhang R, Zeng X, et al. Exposure to neonicotinoid insecticides and their characteristic metabolites: Association with human liver cancer. Environmental Research. Published online May 2022:112703. doi:10.1016/j.envres.2022.112703
- 9.Kannoujia J, Nagineni D, Rodda R, et al. Identification and Characterization of the Isomeric Impurity of the Fungicide “Cyazofamid.” Chemistry An Asian Journal. Published online February 20, 2023. doi:10.1002/asia.202201276
- 10.Li X, Tu M, Yang B, Ma W, Li H. Structurally related impurity profiling of thiacloprid by orbitrap and de novo identification tool. Microchemical Journal. Published online October 2023:109123. doi:10.1016/j.microc.2023.109123
- 11.Ludwig M, Nothias LF, Dührkop K, et al. Database-independent molecular formula annotation using Gibbs sampling through ZODIAC. Nat Mach Intell. Published online October 13, 2020:629-641. doi:10.1038/s42256-020-00234-6





