Sustainable and healthy: Alternative food sources
To meet the challenge of feeding a growing population, sustainable food systems are vital. They have a significant impact on preserving resources and safeguarding biodiversity. While it’s important to focus on strategies like efficient production and reducing waste, promoting healthier eating habits is just as crucial. Healthy diets should include foods high in polyunsaturated fatty acids, especially omega-3 and omega-6 fatty acids, which are essential as the human body cannot make them. They are crucial for brain function, heart and bone health, inflammation reduction and more. They are primarily found in fish and nut oils. There is a growing need to find alternatives to fish oil, due to overfishing and to meet the dietary preferences of vegetarians and vegans.
Nutritious microalgae
Microalgae species, such as Spirulina, Chlorella and Amphora, are tiny but rich in bioactive compounds. They grow under sustainable conditions that conserve marine resources1. With their high content of proteins, carbohydrates, lipids, vitamins and minerals, microalgae offer a valuable nutrient package. They are promoted as healthy food supplements and are available in various forms such as powders, tablets, capsules and liquids2. They are not only consumed by humans but also used as feed for terrestrial and aquatic animals. Spirulina (Arthrospira maxima and Arythrospira platensis) and Chlorella sp. are already widely appreciated3. Spirulina is not only recognized as a “superfood” by the World Health Organization, but also marketed by NASA as the most highly concentrated human food4. Amphora sp. has recently gained attention and has also the potential to be a source of healthy lipids and essential fatty acids5.
| Spirulina (Arthrospira) | Chlorella sp. | Amphora sp. | |
|---|---|---|---|
| classification | – genus of cyanobacteria – dietary supplement is made from A. platensis and A. maxima6 | thirteen species of single-celled green algae of the division Chlorophyta | one of the largest genera of diatoms |
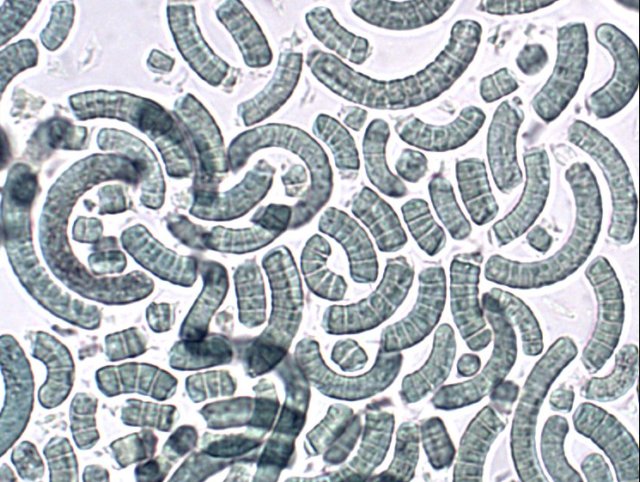 Spirulina powder (Image by John Elson on Wikimedia, CC BY-SA 3.0) | 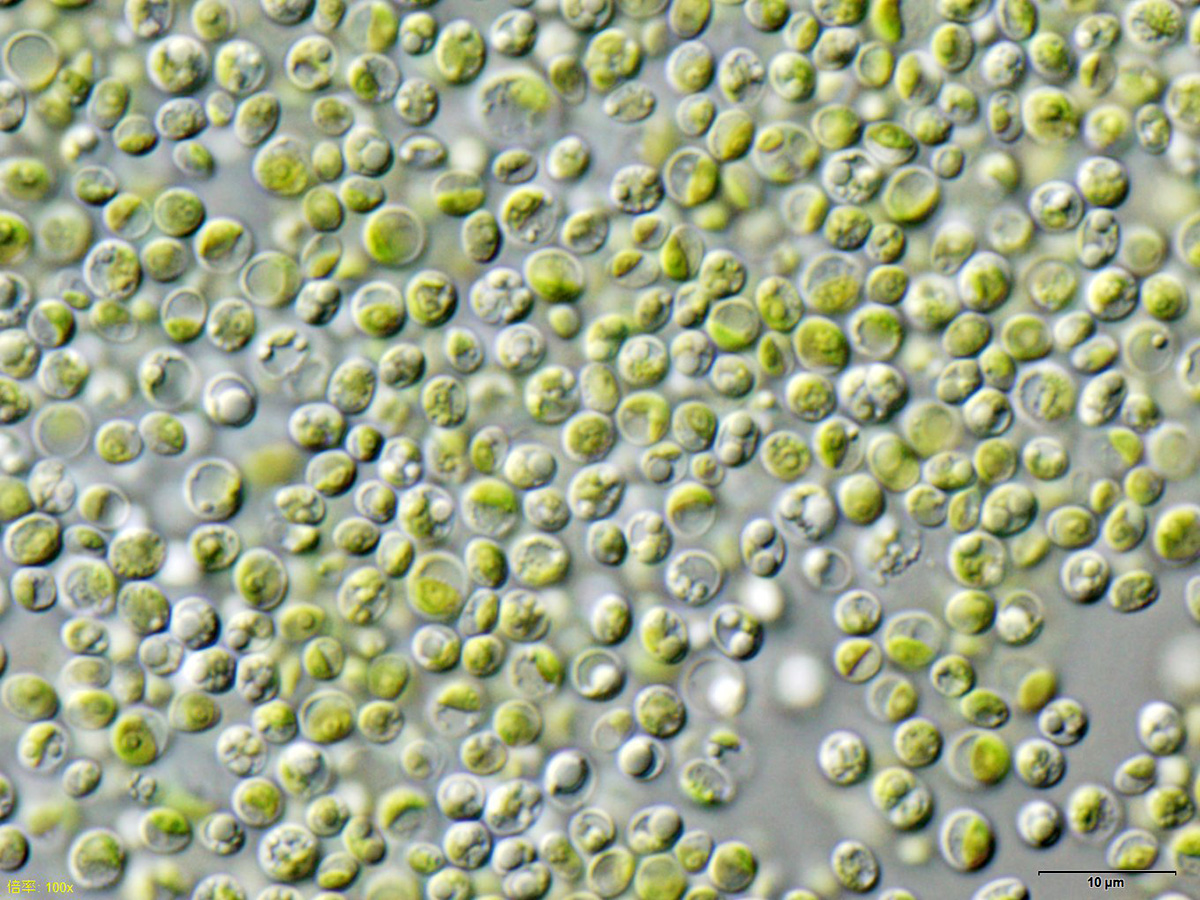 Chlorella vulgaris (Image by user NEON on Wikimedia, CC BY-SA 3.0) | 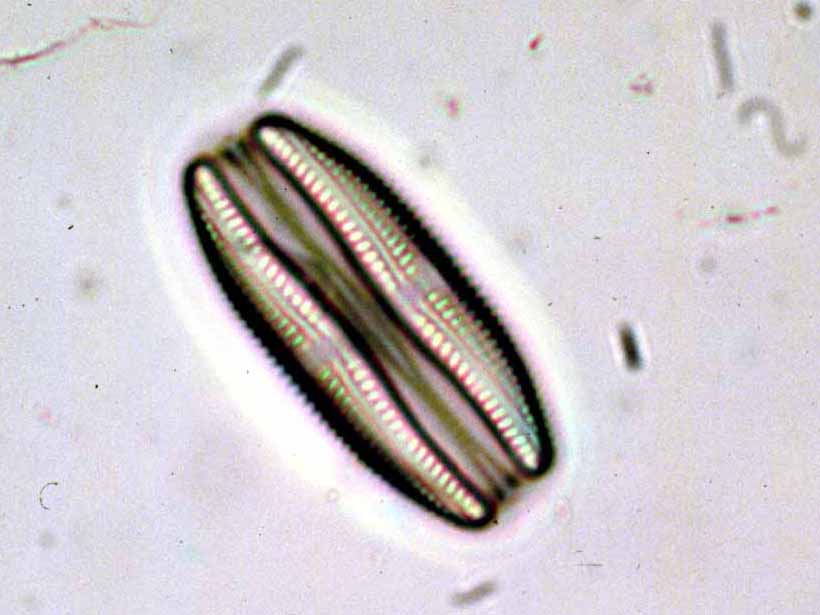 Amphora ovalis (public domain) | |
| morphology | – free-floating filamentous cyanobacteria – cylindrical, multicellular trichomes in an open left-hand helix – various degrees of coiling (tightly-coiled to straight6) – length from 2 to 16 μm | – single-celled green algae – spherical in shape – about 2 to 10 μm in diameter – without flagella – chloroplasts containing chlorophyll-a and -b | – unicellular, oval diatom – 5 to 105 μm long |
| nutritional composition | – very rich in proteins6 – harbours all essential amino acids – high amounts of polyunsaturated fatty acids7 – vitamins, minerals and photosynthetic pigments | – proteins – under certain growing conditions, it yields oils that are high in polyunsaturated fats8 – high lipid content (specially enriched in omega-3 and omega-6 fatty acids)1 | essential lipids and nutritionally important fatty acids5 |
What’s really in it?
Numerous studies have looked at the chemical composition of Spirulina and shed light on its nutritional richness. However, the literature provides relatively little information on their commercial formulations. The reported chemical compositions of microalgae species, including Spirulina, Chlorella and Amphora, show remarkable differences, largely due to differences in cultivation conditions. Understanding these compositional differences is central to recognizing the diverse applications and benefits of microalgae.
Metabolomics for quality control and comparison
While metabolomics is a common tool for the quality control of herbal medicines and the analysis of complex food matrices, its application to marine food supplements is not yet as widespread. Researchers at Cairo University9 are conducting a multiplex metabolomics approach to analyze Spirulina, Chlorella and Amphora to map their metabolome including bioactive compounds and control the quality of Spirulina supplements. Four different commercially available Spirulina platensis samples were analyzed together with samples of Chlorella and Amphora species. To identify their primary and secondary metabolites, the researchers used gas chromatography-mass spectrometry (GC-MS), ultra high performance liquid chromatography coupled with high resolution tandem mass spectrometry (UPLC-HRMS/MS) and ultraviolet-visible spectrophotometry (UV/Vis). They used feature-based molecular networks10 to analyze the relationships between different compounds and SIRIUS + CSI:FingerID 5.6.3 to identify the suspected structures.
Metabolic composition
UPLC-HRMS/MS analysis has revealed the large molecular weight metabolites, including free fatty acids, polar lipids, and pigments. Amphora had a high amount of fatty acids. Spirulina samples were high in glycolipids. And Chlorella had an abundance of phospholipids. These compounds, with their potential health benefits, are increasingly finding their way into novel cuisine.
The “greens”
Porphyrins are photosynthetic pigments that are not only essential for the vitality of microalgae, but also exhibit a spectrum of health-promoting properties, including antimutagenic, antioxidant, chemopreventive and antimicrobial activities11. In addition, they serve as natural food colorants. The researchers found porphyrins to be the predominant pigments in Spirulina, whereas they were only present to a limited extent in Chlorella and Amphora. This is in contradiction to previous findings, which indicate a higher content of photosynthetic pigments in Chlorella12. These discrepancies could be due to different cultivation conditions and extraction protocols.
Primary metabolites: Insights from GC–MS
The researchers used GC–MS to analyse the low molecular weight primary metabolites and found fatty acids/esters and glycosides as the predominant compounds. Spirulina showed an enrichment of palmitic acid, 3-mannobiose and glyceryl glycoside, while Chlorella and Amphora were characterized by a high content of sucrose and leucine, respectively. In particular, GC-MS analysis revealed a higher content of essential amino acids in Chlorella and Amphora, underlining their nutritional richness and suitability as dietary supplements.
Ensuring quality
The study uncovered significant differences in metabolite abundance and composition among Spirulina, Chlorella, and Amphora. The analysis revealed significant variations in the metabolome of Spirulina samples attributed to differences in cultivation settings. This highlights the pressing need to optimize cultivation conditions to ensure consistent quality of microalgae as dietary supplements.
References
- 1.Couto D, Melo T, Conde TA, et al. Food grade extraction of Chlorella vulgaris polar lipids: A comparative lipidomic study. Food Chemistry. Published online May 2022:131685. doi:10.1016/j.foodchem.2021.131685
- 2.Koyande AK, Chew KW, Rambabu K, Tao Y, Chu DT, Show PL. Microalgae: A potential alternative to health supplementation for humans. Food Science and Human Wellness. Published online March 2019:16-24. doi:10.1016/j.fshw.2019.03.001
- 3.Sathasivam R, Radhakrishnan R, Hashem A, Abd_Allah EF. Microalgae metabolites: A rich source for food and medicine. Saudi Journal of Biological Sciences. Published online May 2019:709-722. doi:10.1016/j.sjbs.2017.11.003
- 4.Sorrenti V, Castagna DA, Fortinguerra S, Buriani A, Scapagnini G, Willcox DC. Spirulina Microalgae and Brain Health: A Scoping Review of Experimental and Clinical Evidence. Marine Drugs. Published online May 22, 2021:293. doi:10.3390/md19060293
- 5.Hogan P, Otero P, Murray P, Saha SK. Effect of biomass pre-treatment on supercritical CO2 extraction of lipids from marine diatom Amphora sp. and its biomass evaluation as bioethanol feedstock. Heliyon. Published online January 2021:e05995. doi:10.1016/j.heliyon.2021.e05995
- 6.Ciferri O. Spirulina, the edible microorganism. Microbiol Rev. Published online December 1983:551-578. doi:10.1128/mr.47.4.551-578.1983
- 7.Spolaore P, Joannis-Cassan C, Duran E, Isambert A. Commercial applications of microalgae. Journal of Bioscience and Bioengineering. Published online February 2006:87-96. doi:10.1263/jbb.101.87
- 8.Yongmanitchai W, Ward OP. Growth of and omega-3 fatty acid production by Phaeodactylum tricornutum under different culture conditions. Appl Environ Microbiol. Published online February 1991:419-425. doi:10.1128/aem.57.2.419-425.1991
- 9.Hegazi N, Khattab AR, Saad HH, Abib B, Farag MA. A multiplex metabolomic approach for quality control of Spirulina supplement and its allied microalgae (Amphora & Chlorella) assisted by chemometrics and molecular networking. Sci Rep. Published online February 2, 2024. doi:10.1038/s41598-024-53219-5
- 10.Nothias LF, Petras D, Schmid R, et al. Feature-based molecular networking in the GNPS analysis environment. Nat Methods. Published online August 24, 2020:905-908. doi:10.1038/s41592-020-0933-6
- 11.Lafarga T, Fernández-Sevilla JM, González-López C, Acién-Fernández FG. Spirulina for the food and functional food industries. Food Research International. Published online November 2020:109356. doi:10.1016/j.foodres.2020.109356
- 12.Hynstova V, Sterbova D, Klejdus B, Hedbavny J, Huska D, Adam V. Separation, identification and quantification of carotenoids and chlorophylls in dietary supplements containing Chlorella vulgaris and Spirulina platensis using High Performance Thin Layer Chromatography. Journal of Pharmaceutical and Biomedical Analysis. Published online January 2018:108-118. doi:10.1016/j.jpba.2017.09.018





