The chemical diversity of liverworts
Metabolites from bryophytes have received increased attention in recent years due to their potential cytotoxicity against human cancer cell lines1, herbicidal activity2, and fungicidal activity3. In particular one group of bryophytes, the liverworts, are incredibly chemically diverse despite their relatively simple physical morphology. They have unique cell organelles, so-called oil bodies, that are responsible for the synthesis and storage of most specialized metabolites produced by liverworts and are thought to be crucial for defense against herbivory4.
Untarget metabolomics
Special metabolites are often produced under stress. A group of researchers led by Kristian Peters from the Leibniz Institute of Plant Biochemistry studied the metabolic stress response of the leaf liverwort Radula complanata5. They exposed the plant to two growth-promoting hormones and three phytohormones for the stress response. They used untargeted metabolomics6 to analyze the full range of metabolic change without isolating individual metabolites.
The samples were separated using Bruker Elite HPLC coupled to a Bruker TIMS-TOF for measurement of MS1 spectra in positive and negative mode with electrospray ionization. Fragmentation spectra were measured in data-dependent acquisition mode, also referred to as automatic MS/MS. The researchers uploaded the set of spectra to MetaboLights7, a publicly available repository for metabolomics data.
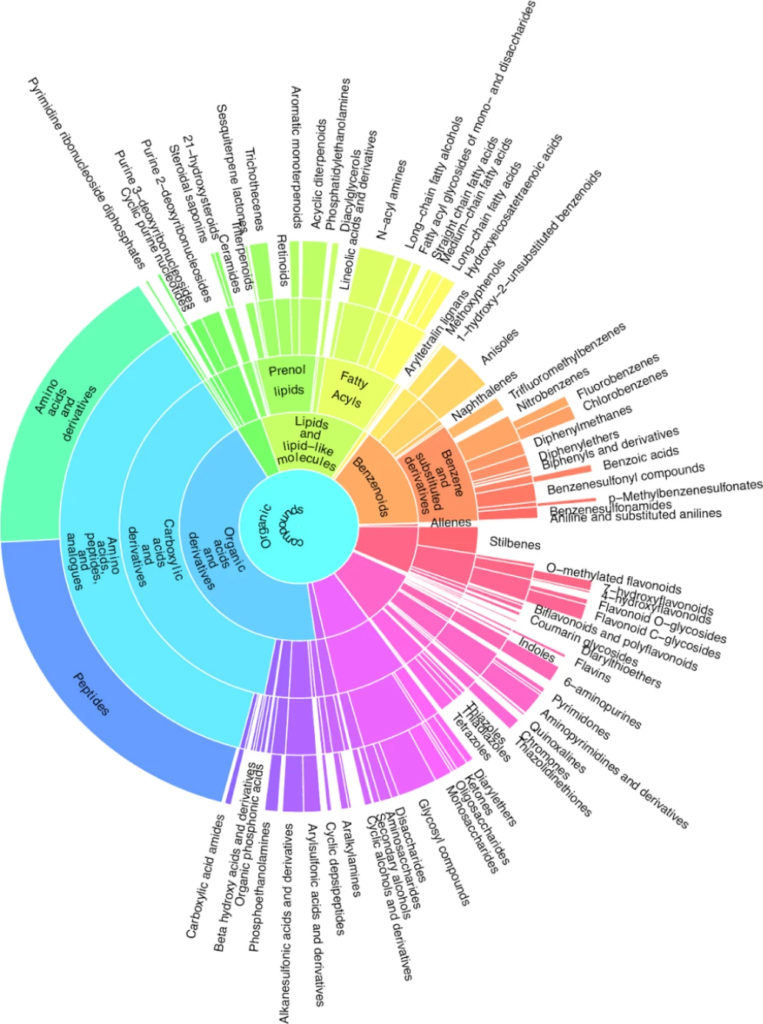
Metabolite classification with CANOPUS
The researchers annotated the fragmentation spectra with SIRIUS8. First, they determined the molecular formulas with ZODIAC9, then predicted the molecular fingerprints with CSI:FingerID10 and used CANOPUS11 to assign the compound class based on the ClassyFire12 and NPClassifier13 ontologies. They only considered molecular formulas from natural product-based databases. In total, our software SIRIUS annotated 211 compound classes under the described conditions. The researchers illustrated the diversity of compound classes in a sunburst plot. An interactive, zoomable representation of the plot is available on Zenodo.
Metabolic stress response
Hormone treatments have only slightly affected the metabolism of R. complanata at a global level. Using statistical methods, the researchers examined the metabolic shifts. Of the 91 metabolites identified that fluctuate under the effects of phytohormones, 71 have been classified with CANOPUS. (Please note that CANOPUS actually returns a compound class for each submitted compound. Unclassified compounds might be due to missing or low quality fragmentation spectra.) Of these, 30 metabolites belong to primary metabolism and 27 to specialized metabolism (the remaining classes were too broad to be constrained to a specific metabolic type). Stress hormones largely downregulated primary metabolites and increased production of specialized metabolites. In contrast, growth hormones largely upregulated primary metabolite production and downregulated stress response metabolites.
To map the functional relationships and biochemical pathways of these compounds, the researchers also created a molecular network. Most of the specialized metabolic pathways are involved in defense and are stimulated by stress hormone pathways. In liverworts, many of these specialized metabolites are found in the oil bodies, which are critical for herbivore defense, or have been identified as antifungal and are produced during fungal infections3. These results are consistent with stress responses of other moss species, but differ from results for vascular plants, highlighting the unique metabolic processes of liverworts.
References
- 1.Dey A, Mukherjee A. Therapeutic potential of bryophytes and derived compounds against cancer. Journal of Acute Disease. Published online August 2015:236-248. doi:10.1016/j.joad.2015.04.011
- 2.Zhang CY, Gao Y, Zhu RX, et al. Prenylated Bibenzyls from the Chinese Liverwort Radula constricta and Their Mitochondria-Derived Paraptotic Cytotoxic Activities. J Nat Prod. Published online July 3, 2019:1741-1751. doi:10.1021/acs.jnatprod.8b00897
- 3.Commisso M, Guarino F, Marchi L, Muto A, Piro A, Degola F. Bryo-Activities: A Review on How Bryophytes Are Contributing to the Arsenal of Natural Bioactive Compounds against Fungi. Plants. Published online January 21, 2021:203. doi:10.3390/plants10020203
- 4.Kanazawa T, Morinaka H, Ebine K, et al. The liverwort oil body is formed by redirection of the secretory pathway. Nat Commun. Published online December 1, 2020. doi:10.1038/s41467-020-19978-1
- 5.Blatt-Janmaat K, Neumann S, Schmidt F, Ziegler J, Qu Y, Peters K. Impact of in vitro phytohormone treatments on the metabolome of the leafy liverwort Radula complanata (L.) Dumort. Metabolomics. Published online March 9, 2023. doi:10.1007/s11306-023-01979-y
- 6.Peters K, Balcke G, Kleinenkuhnen N, Treutler H, Neumann S. Untargeted In Silico Compound Classification—A Novel Metabolomics Method to Assess the Chemodiversity in Bryophytes. IJMS. Published online March 23, 2021:3251. doi:10.3390/ijms22063251
- 7.Haug K, Salek RM, Conesa P, et al. MetaboLights—an open-access general-purpose repository for metabolomics studies and associated meta-data. Nucleic Acids Research. Published online October 29, 2012:D781-D786. doi:10.1093/nar/gks1004
- 8.Dührkop K, Fleischauer M, Ludwig M, et al. SIRIUS 4: a rapid tool for turning tandem mass spectra into metabolite structure information. Nat Methods. Published online March 18, 2019:299-302. doi:10.1038/s41592-019-0344-8
- 9.Ludwig M, Nothias LF, Dührkop K, et al. Database-independent molecular formula annotation using Gibbs sampling through ZODIAC. Nat Mach Intell. Published online October 13, 2020:629-641. doi:10.1038/s42256-020-00234-6
- 10.Dührkop K, Shen H, Meusel M, Rousu J, Böcker S. Searching molecular structure databases with tandem mass spectra using CSI:FingerID. Proc Natl Acad Sci USA. Published online September 21, 2015:12580-12585. doi:10.1073/pnas.1509788112
- 11.Dührkop K, Nothias LF, Fleischauer M, et al. Systematic classification of unknown metabolites using high-resolution fragmentation mass spectra. Nat Biotechnol. Published online November 23, 2020:462-471. doi:10.1038/s41587-020-0740-8
- 12.Djoumbou Feunang Y, Eisner R, Knox C, et al. ClassyFire: automated chemical classification with a comprehensive, computable taxonomy. J Cheminform. Published online November 4, 2016. doi:10.1186/s13321-016-0174-y
- 13.Kim HW, Wang M, Leber CA, et al. NPClassifier: A Deep Neural Network-Based Structural Classification Tool for Natural Products. J Nat Prod. Published online October 18, 2021:2795-2807. doi:10.1021/acs.jnatprod.1c00399








